Proteins – poly-peptide chains.
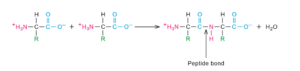
Proteins are the most versatile molecules of natural bio molecular plans. From a chemical point of view, proteins are the most structurally complex and functionally sophisticated molecules which are made up of twenty amino acids with different chemical properties joining with peptide bonds. Proteins are therefore known as polypeptides. Each type of protein has unique set of amino acids and there are many thousands of different proteins. In proteins amino acids are joined together by linkages called peptide bonds. The formation of peptide bond is a condensation reaction since the process release a water molecule. Peptide bond has partial double bond character.
Formation of the peptide bond.
There can be dipeptides, oligopeptides and polypeptides which are named considering the number of amino acids contribute for the formation of the polypeptide chain.
Proteins are made by folding of the polypeptide chain to form a specific shape. Even though every protein is a polypeptide chain, every polypeptide is not a protein since a protein should have a properly folded conformation.
[g_article_ads]
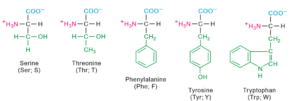
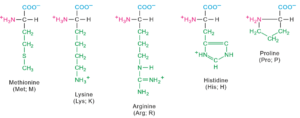
Twenty amino acids contribute for the formation of proteins.
Structure of proteins.
The structure of protein is extremely complicated. There are four types of structures.
Primary structure.
The primary structure of the protein is the linear sequence of amino acids joined together by peptide bonds.
Secondary structure.
The regular folding of regions of the polypeptide chain forms the secondary structure. There are three types of folding patterns.
- Alpha helices.
- Beta pleated sheets.
- Beta turns (bends).
The secondary structures are stabilized by hydrogen bonding between neighboring amino acids. These hydrogen bonds are responsible for alpha helices.
Beta turns are bendings of polypeptide chains. They reverse the direction of the polypeptide chain. The only amino acids often found in these regions are glycine and proline because,
- Glycine is a small flexible molecule and it can be accommodated easily where other amino acids cannot.
- The nitrogen atom of protein cannot usually form hydrogen bond so it produces a bend.
The alpha helices and beta pleated sheets are the most easily recognized secondary structures in a wide variety of proteins
Tertiary structure.
This is a special arrangement of amino acids that are far apart in the linear sequence as well as those residues that are adjacent. A special relationship between secondary structural elements to a produce more compact tridimensional shape. In tertiary structures, there are several interactions other than the hydrogen bonds like disulfide bonds, vanderwaals interactions, electrostatic interactions etc.
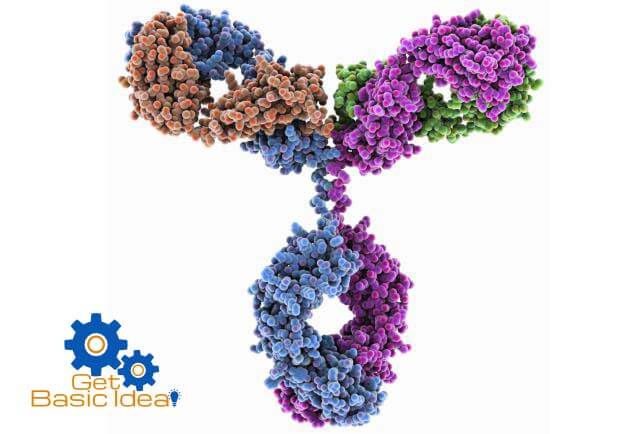
Quaternary structure.
The quaternary structure of proteins is formed by the special arrangements of the polypeptide subunits due to the interactions between them. Therefore proteins containing more than one polypeptide chain exhibit a forth level of protein structure called quaternary structure.
The interactions between among the polypeptide subunits may be covalent links or non-covalent interactions.
Ex:
- Peptide bonds.
- Hydrogen bonds.
- Hydrophobic forces.
- Electrostatic alterations.
- Vandewaals interactions.
- Disulfide bonds.
There can be intrachain disulfide bonds and interchain disulfide bonds in the quaternary structure of proteins.
The proteins play roles virtually in all biological processes.
Functions of proteins.
[g_article_ads]
-
Functional- enzymes and hormones :
Virtually all the chemical reactions of organic biomolecules are catalyzed by enzymes. They are the most varied and most highly specialized proteins with a catalytic activity.
Some proteins help to regulate cellular or physiological activities.
Ex:
- Insulin
- Growth hormone (GH)
- FSH
- LH
-
Transport and storage :
Transport proteins present on blood plasma bind and carry specific molecules or ions from one organ to another.
Ex:
- Hemoglobin
- Myoglobin
- Ferritin
-
Structural :
Proteins act as a structural material.
Ex:
- Collagen
- keratin
-
Coordinate motion- muscle proteins.
Ex:
- Actin
- Myosin
-
Control of growth.
Ex:
- Growth factors
- repressors
-
Immune protection- antibodies.
Many proteins defend organisms against invasion by other species.
Ex:
- immunoglobulin
- fibrinogen
- thrombin
-
Sensory functions.
Some animals have proteins which is sensitive some extreme conditions.
Animals living in Polar Regions have antifreeze proteins which help to survive without freezing.
Admin of Get Basic Idea / Senior Solution Architect.


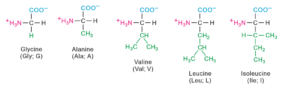
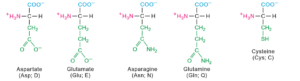
After study a few of the content for your website now, i genuinely as if your technique of blogging. I bookmarked it to my bookmark website list and are checking back soon. Pls have a look at my website too and told me if you agree.