There are two proposed models for explain the enzyme substrate interactions.
- Lock and key model
- Induced fit model
1. Lock and key model.
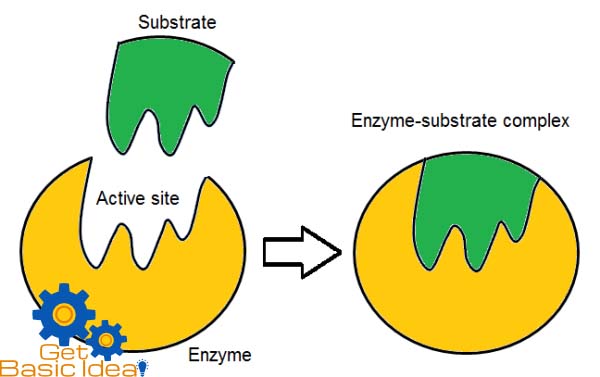
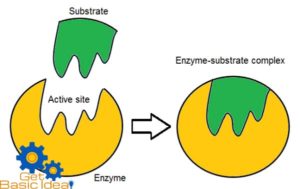
This model is proposed by Emil Fisher in 1984 as a result of studies carried out on enzyme specificity. This hypothesis states that the enzymes are structurally complementary to their substrates, as they can fit together like a lock and key. Active sites of the enzyme is perfectly shaped space for the shape of the substrate.
[g_article_ads]
According to this hypothesis active sites of the enzymes are inflexible hence cannot change their shape complementary to variety of substrates. They can bind only with a particular type of substrate having complementary shape. So the enzymes are specific to their substrates. This is known as the enzyme – substrate.
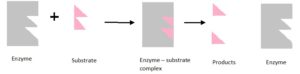
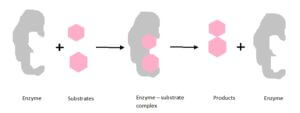
The substrates bind with the active sites having complementary shape on the enzyme. Then the enzyme – substrate is formed and reaction is performed resulting the final products. Enzymes are not consumed during the reactions and can participate again for catalyze the reaction.
The results of some experiments could not be explained using this key and lock model. So in 1958, Daniel Koshland suggested a modification for lock and key model.
2 .Induced fit model.
[g_article_ads]
This model is a further modification of the lock and key model. It states that the active sites of the enzymes are more flexible than it was thought. These active sites continuously change with the substrate as substrate interact with the enzyme. Substrate fits in to a general shape of the active site in the enzyme. This is not a perfect fit. Substrate – enzyme interaction cause a shape change in the active site. The change of protein configuration leads to near perfect fit of enzyme to the substrate.
Example for induced fit model – Glucose hexokinase activity.
Glucose hexokinase is an enzyme that catalyzes the ATP dependent phosphorylation of glucose and form glucose – 6 phosphate, When glucose binds to the active site of the glucose hexokinase enzyme,
Non covalent interactions take place between the enzyme and substrate. The shape of the active site is changed which then fit perfectly to the substrate transition state. Glucose hexokinase put the glucose molecule to correct orientation to make the reaction proceed. The catalysis is stated by the active sites of the enzyme. Especially R groups of the amino acids play a vital role in catalysis.
The transition state of the glucose / glucose -6- phosphate is formed due to the orientation of the glucose and releasing of the binding energy. The transition state is highly energetic compared to substrate or to the product. Therefor this has to be held more tightly to make it stabilize.
When reaction proceed, the phosphate bond is formed and glucose -6- phosphate is formed as a result. Glucose -6- phosphate is no longer fits to the active site hence it is released from the enzyme.
Admin of Get Basic Idea / Senior Solution Architect.

My developer is trying to convince me to move to .net from PHP. I have always disliked the idea because of the expenses. But he's tryiong none the less. I've been using WordPress on several websites for about a year and am nervous about switching to another platform. I have heard very good things about blogengine.net. Is there a way I can transfer all my wordpress content into it? Any help would be greatly appreciated!
Hey! Do you know if they make any plugins to safeguard against hackers? I'm kinda paranoid about losing everything I've worked hard on. Any tips?
Thanks for the marvelous posting! I definitely enjoyed reading it, you might be a great author.I will make certain to bookmark your blog and will often come back down the road. I want to encourage yourself to continue your great job, have a nice evening!
Wonderful, what a webpage it is! This website provides useful facts to us, keep it up.
It's actually a cool and useful piece of info. I am satisfied that you simply shared this helpful information with us. Please stay us up to date like this. Thank you for sharing.
Have you ever considered creating an e-book or guest authoring on other blogs? I have a blog based upon on the same ideas you discuss and would really like to have you share some stories/information. I know my viewers would value your work. If you are even remotely interested, feel free to shoot me an e mail.
What's up, just wanted to say, I liked this post. It was funny. Keep on posting!
Hello, I do believe your website could possibly be having browser compatibility problems. Whenever I take a look at your blog in Safari, it looks fine however, if opening in I.E., it's got some overlapping issues. I merely wanted to provide you with a quick heads up! Besides that, excellent website!
I am regular visitor, how are you everybody? This piece of writing posted at this web site is really pleasant.
That is very interesting, You are a very skilled blogger. I have joined your feed and stay up for in the hunt for more of your excellent post. Additionally, I have shared your web site in my social networks
Hi there, just wanted to say, I liked this article. It was inspiring. Keep on posting!
Hey There. I discovered your blog the usage of msn. This is a really smartly written article. I will be sure to bookmark it and come back to learn extra of your helpful information. Thank you for the post. I'll definitely return.
Thank you for the auspicious writeup. It in fact was a amusement account it. Look advanced to far added agreeable from you! However, how can we communicate?
Hey! This is my first comment here so I just wanted to give a quick shout out and say I truly enjoy reading through your blog posts. Can you recommend any other blogs/websites/forums that go over the same subjects? Thanks a ton!|
Great post. I was checking constantly this weblog and I am inspired! Very useful info specially the ultimate part 🙂 I handle such information much. I was looking for this certain information for a very lengthy time. Thank you and best of luck.
Hello, Neat post. There is an issue together with your site in web explorer, might test this? IE still is the marketplace leader and a big portion of folks will pass over your wonderful writing because of this problem.
Wow, fantastic weblog layout! How long have you ever been blogging for? you make running a blog look easy. The entire glance of your web site is great, as neatly as the content!